

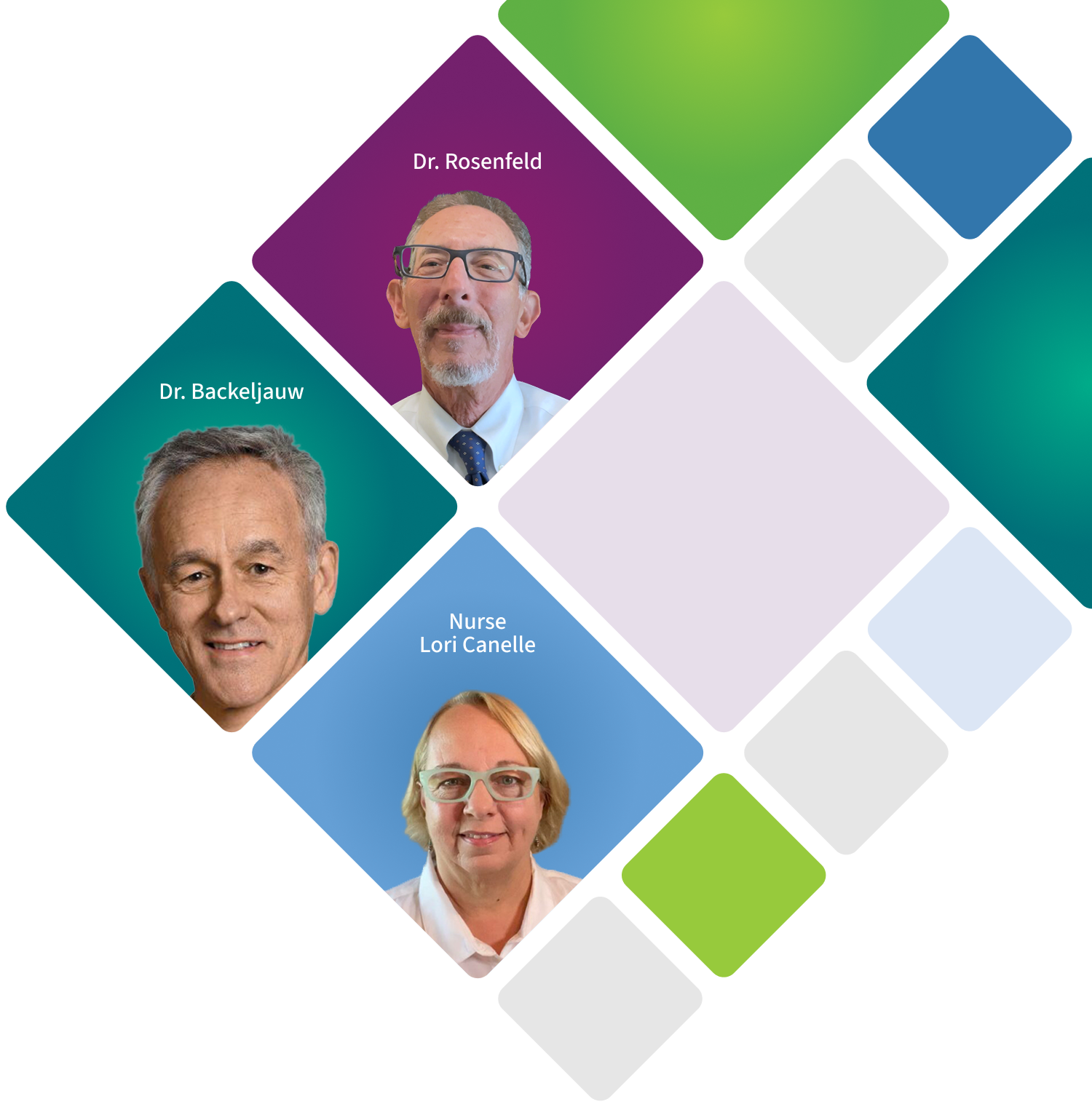

Meet the
Hear from healthcare professionals, patients, and caregivers as they share their expertise and experience on INCRELEX® (mecasermin).
On this page
Learn From the IncrelEXPERTS®
The IncrelEXPERTS® are a network of healthcare professionals, patients, and caregivers committed to sharing their knowledge about INCRELEX, the only treatment option for severe primary IGF-1 deficiency (SPIGFD). Based on real-world experience, you’ll learn how to recognize and diagnose SPIGFD, dose and titrate INCRELEX, and more. Explore these topics by watching the videos below.
Get to Know the IncrelEXPERTS®

IncrelEXPERT
Meet Philippe Backeljauw, MD
Dr. Philippe Backeljauw is a Pediatric Endocrinologist at Cincinnati Children’s Medical Center who specializes in growth disorders.
Recognized internationally as an expert in growth hormone resistance, Dr. Backeljauw is passionate about improving the process of diagnosis and treating patients with growth disorders.
Years of spearheading research has led to hundreds of peer-reviewed manuscripts and abstracts, and contributed greatly to the treatment landscape of pediatric growth disorders

IncrelEXPERT
Meet Ron Rosenfeld, MD
Dr. Rosenfeld is a professor of pediatrics emeritus at Oregon Health & Science University and at Stanford University.
Internationally renowned for his contributions to uncovering the role of growth factors and their receptors in growth disorders like SPIGFD.
Dedication to research and understanding of the biology of growth hormone and growth factors has earned Dr. Rosenfeld numerous awards.

IncrelEXPERT
Meet Lori Casnellie, RN
Disease Education
ASK AN IncrelEXPERT
The Role of GH and IGF-1 to Promote Growth
ASK AN IncrelEXPERT
Clinical Characteristics of Severe Primary IGF-1 Deficiency (SPIGFD)
Sign-up for information and updates
Diagnosis
ASK AN IncrelEXPERT
Diagnostic Criteria for Severe Primary IGF-1 Deficiency (SPIGFD)
ASK AN IncrelEXPERT
Diagnosing Severe Primary IGF-1 Deficiency (SPIGFD)
Sign-up for information and updates
Dosing
ASK AN IncrelEXPERT
3 Steps to Dosing INCRELEX
Sign-up for information and updates
Tips for Nurses
ASK AN IncrelEXPERT
Care Team
ASK AN IncrelEXPERT
Counseling
Nurse Lori provides counseling tips for INCRELEX patients and their caregivers.
Sign-up for information and updates
Indication and important safety information
INCRELEX® (mecasermin) is indicated for the treatment of growth failure in pediatric patients aged 2 years and older with severe primary IGF-1 deficiency* (IGFD), or with hormone (GH) gene deletion who have developed neutralizing antibodies to GH.
Limitations of use: INCRELEX is not a substitute to GH for approved GH indications. INCRELEX is not indicated for use in patients with secondary forms of IGFD, such as GH deficiency, malnutrition, hypothyroidism, or chronic treatment with pharmacologic doses of anti-inflammatory steroids.
*Severe primary IGF-1 deficiency (IGFD) is defined by height standard deviation score ≤ -3.0 and basal IGF-1 standard deviation score ≤ -3.0 and normal or elevated GH.
Important safety information
Contraindications
- Hypersensitivity: to mecasermin (rhIGF-1), any of the inactive ingredients in INCRELEX, or who have experienced a severe hypersensitivity to INCRELEX. Allergic reactions have been reported, including anaphylaxis requiring hospitalization.
- Closed Epiphyses
- Malignant Neoplasia in pediatric patients with malignant neoplasia or a history of malignancy.
Warning and precautions
- Hypoglycemia: INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is omitted. Glucose monitoring and INCRELEX dose titration are recommended until a well-tolerated dose is established and as medically indicated.
- Intracranial Hypertension: Funduscopic examination is recommended at the initiation of and periodically during the course of therapy.
- Lymphoid Tissue Hypertrophy: Patients should have periodic examinations to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients receiving products indicated to treat growth failure and/or short stature, including INCRELEX. Carefully evaluate any pediatric patient with the onset of a limp or hip/knee pain during INCRELEX therapy.
- Progression of Scoliosis: Patients with a history of scoliosis, treated with INCRELEX, should be monitored.
- Malignant Neoplasia: There have been postmarketing reports of malignant neoplasia in pediatric patients who received treatment with INCRELEX. It is unknown whether there is any relationship between Increlex therapy and new occurrences of neoplasia. The tumors were observed more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced serum IGF-1 levels above the normal reference ranges for age and sex. Monitor all patients receiving INCRELEX carefully for development of neoplasms. If malignant neoplasia develops, discontinue INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
Adverse reactions
Common adverse reactions include hypoglycemia, local and systemic hypersensitivity, and tonsillar hypertrophy.
To report a suspected adverse event related to INCRELEX, contact Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
Indication and important safety information
INCRELEX® (mecasermin) is indicated for the treatment of growth failure in pediatric patients aged 2 years and older with severe primary IGF-1 deficiency* (IGFD), or with hormone (GH) gene deletion who have developed neutralizing antibodies to GH.
Limitations of use: INCRELEX is not a substitute to GH for approved GH indications. INCRELEX is not indicated for use in patients with secondary forms of IGFD, such as GH deficiency, malnutrition, hypothyroidism, or chronic treatment with pharmacologic doses of anti-inflammatory steroids.
*Severe primary IGF-1 deficiency (IGFD) is defined by height standard deviation score ≤ -3.0 and basal IGF-1 standard deviation score ≤ -3.0 and normal or elevated GH.
Important safety information
Contraindications
- Hypersensitivity: to mecasermin (rhIGF-1), any of the inactive ingredients in INCRELEX, or who have experienced a severe hypersensitivity to INCRELEX. Allergic reactions have been reported, including anaphylaxis requiring hospitalization.
- Closed Epiphyses
- Malignant Neoplasia in pediatric patients with malignant neoplasia or a history of malignancy.
Warning and precautions
- Hypoglycemia: INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is omitted. Glucose monitoring and INCRELEX dose titration are recommended until a well-tolerated dose is established and as medically indicated.
- Intracranial Hypertension: Funduscopic examination is recommended at the initiation of and periodically during the course of therapy.
- Lymphoid Tissue Hypertrophy: Patients should have periodic examinations to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients receiving products indicated to treat growth failure and/or short stature, including INCRELEX. Carefully evaluate any pediatric patient with the onset of a limp or hip/knee pain during INCRELEX therapy.
- Progression of Scoliosis: Patients with a history of scoliosis, treated with INCRELEX, should be monitored.
- Malignant Neoplasia: There have been postmarketing reports of malignant neoplasia in pediatric patients who received treatment with INCRELEX. It is unknown whether there is any relationship between Increlex therapy and new occurrences of neoplasia. The tumors were observed more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced serum IGF-1 levels above the normal reference ranges for age and sex. Monitor all patients receiving INCRELEX carefully for development of neoplasms. If malignant neoplasia develops, discontinue INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
Adverse reactions
Common adverse reactions include hypoglycemia, local and systemic hypersensitivity, and tonsillar hypertrophy.
To report a suspected adverse event related to INCRELEX, contact Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
