
INCRELEX® to Treat Severe Primary IGF-1 Deficiency
On this page

Olive, a former INCRELEX patient, at age 18, and her mother, Renee.
Mechanism of Action
Established Safety Profile With Increlex
Adverse reactions occurring with Increlex in ≥5% of patients in clinical studies included* 1:
- Hypoglycemia, lipohypertrophy, bruising, otitis media, serous otitis media, snoring, tonsillar hypertrophy, headache, dizziness, convulsions, vomiting, hypoacusis, fluid in middle ear, ear pain, abnormal tympanometry, cardiac murmur, arthralgia, pain in extremity, thymus hypertrophy, ear tube insertion
Hypoglycemia was reported by 30 patients (42%) at least once during their course of therapy 1
- Most cases of hypoglycemia were mild or moderate in severity
- Five subjects had severe hypoglycemia (requiring assistance and treatment) on one or more occasions and 4 subjects experienced hypoglycemic seizures/loss of consciousness on one or more occasions
- 14 of the 30 patients (47%) reporting hypoglycemia had a history of hypoglycemia prior to treatment
- The frequency of hypoglycemia was highest in the first month of treatment and episodes were more frequent in younger children
- Symptomatic hypoglycemia was generally avoided when a meal or snack was consumed either shortly (i.e., 20 minutes) before or after the administration of INCRELEX®
No patients withdrew from any clinical study because of adverse reactions. 1
Improve Growth Rates in Children With Severe Primary IGF-1 Deficiency
- The average height SDS was -6.7 at baseline for the 61 subjects in the efficacy analysis.
- Mean height velocity increased to 8 cm/year in the first year, on average, from a baseline of 2.8 cm/year (P<0.0001).
- Mean height velocity was sustained at approximately 5 cm/year in Years 2 through 6 of treatment.
- Forty-nine subjects were included in an analysis of the effects of INCRELEX® on bone age advancement.
- The mean ± standard deviation (SD) change in chronological age was 4.9 ± 3.4 years.
- The mean ± SD change in bone age was 5.3 ± 3.4 years.
When Does Primary IGFD Become Severe Primary IGFD?
After ruling out other diseases and inadequate nutrition, growth failure caused by SPIGFD is defined by 7:
- height standard deviation score (SDS) ≤ -3.0
- basal IGF-1 SDS ≤ -3.0
- normal or elevated GH
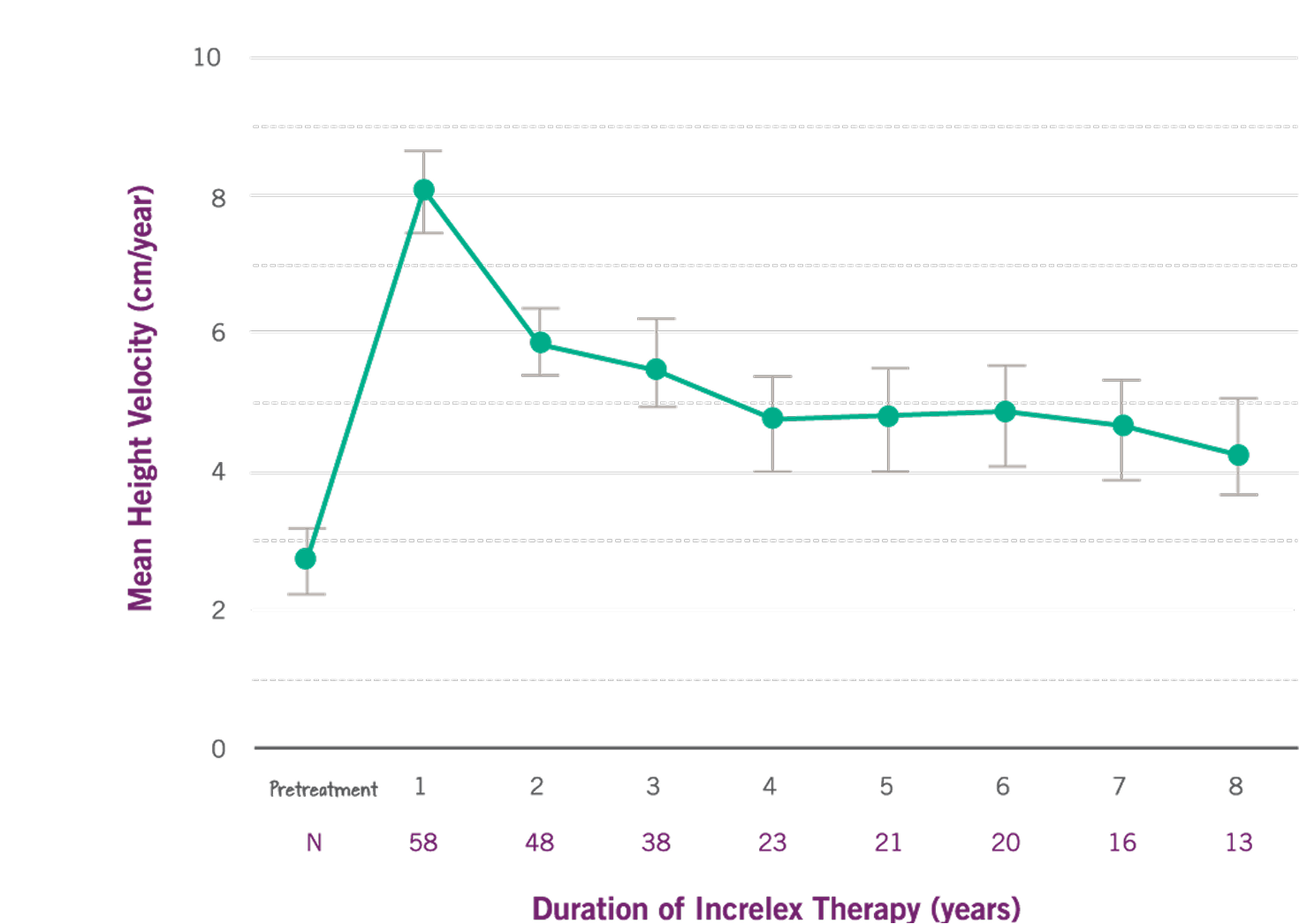
Growth Rate with INCRELEX® over 8 years 1
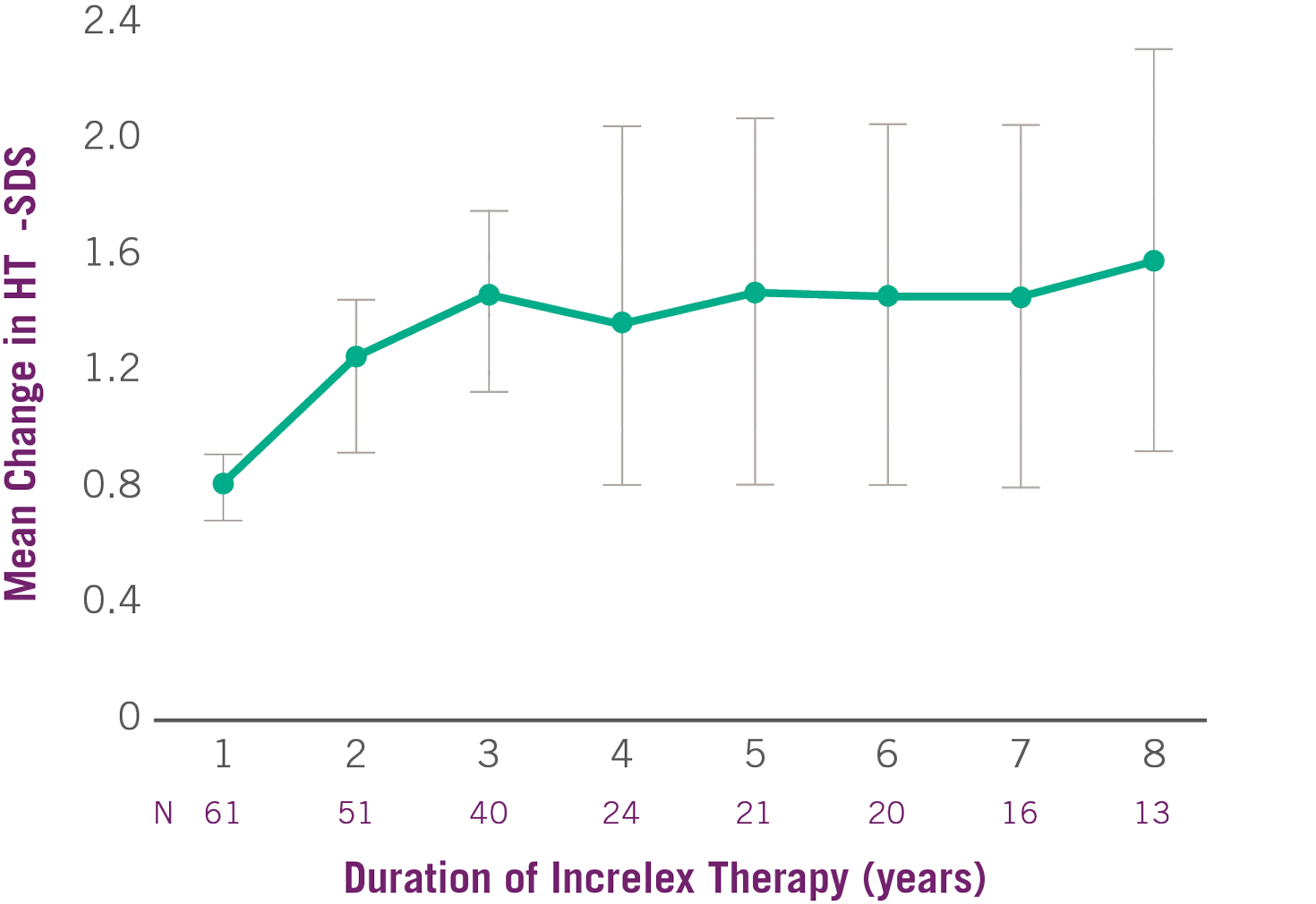
Change in Height SDS with INCRELEX® Over 8 Years 1
INCRELEX® Dosing and Administration
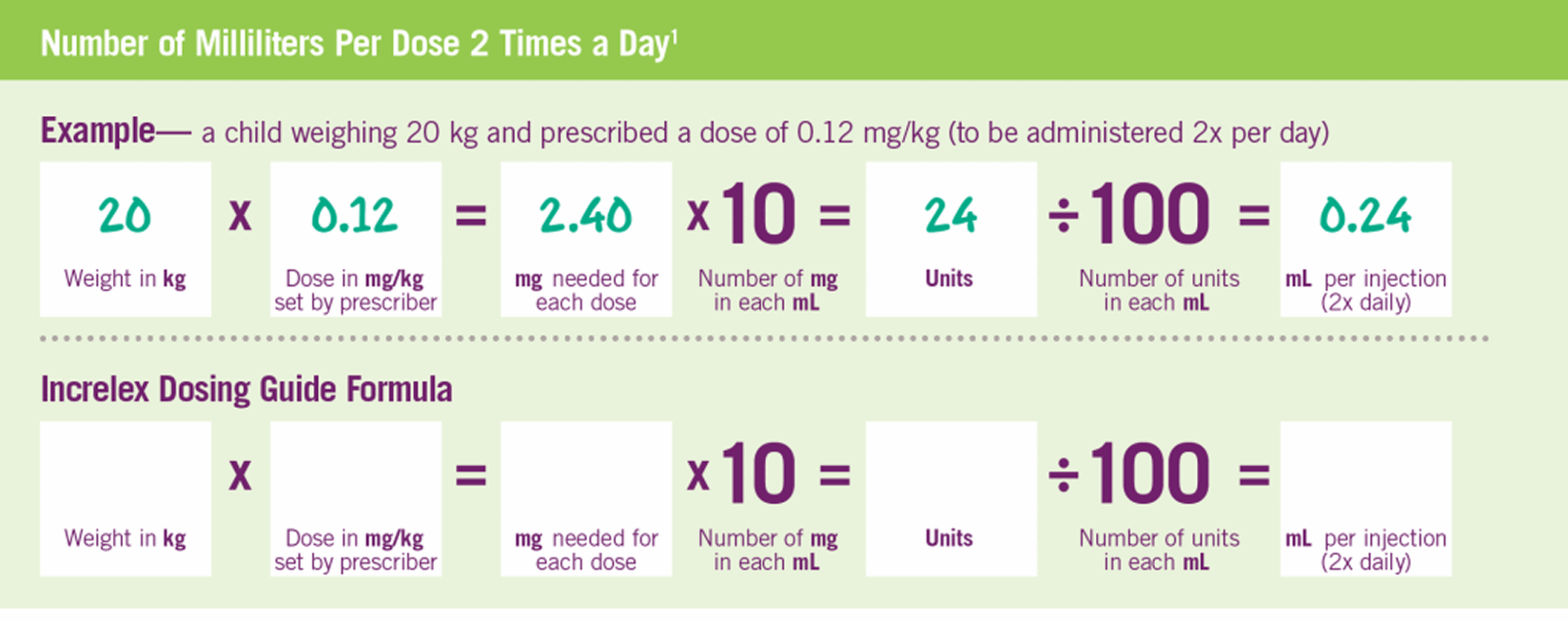
INCRELEX® Dosing is Weight-based1
Pre-prandial glucose monitoring is recommended at treatment initiation and until a well-tolerated dose is established. If frequent symptoms of hypoglycemia or severe hypoglycemia occur, pre-prandial glucose monitoring should continue. If hypoglycemia occurs with recommended doses despite adequate food intake, the dose should be reduced.1

Three Simple Steps to INCRELEX® Dosing1
Adapted from INCRELEX full prescribing information.1 BID, twice daily. *Doses greater than 0.12 mg/kg given BID have not been evaluated in children with primary IGFD and, due to the potential risk of neoplasia and hypoglycaemic effects, should not be used. If hypoglycemia occurs with recommended doses despite adequate food intake, the dose should be reduced.1 Pre-prandial glucose monitoring is recommended at treatment initiation and until a well-tolerated dose is established.1 If frequent symptoms of hypoglycemia or severe hypoglycemia occur, pre-prandial glucose monitoring should continue. 1
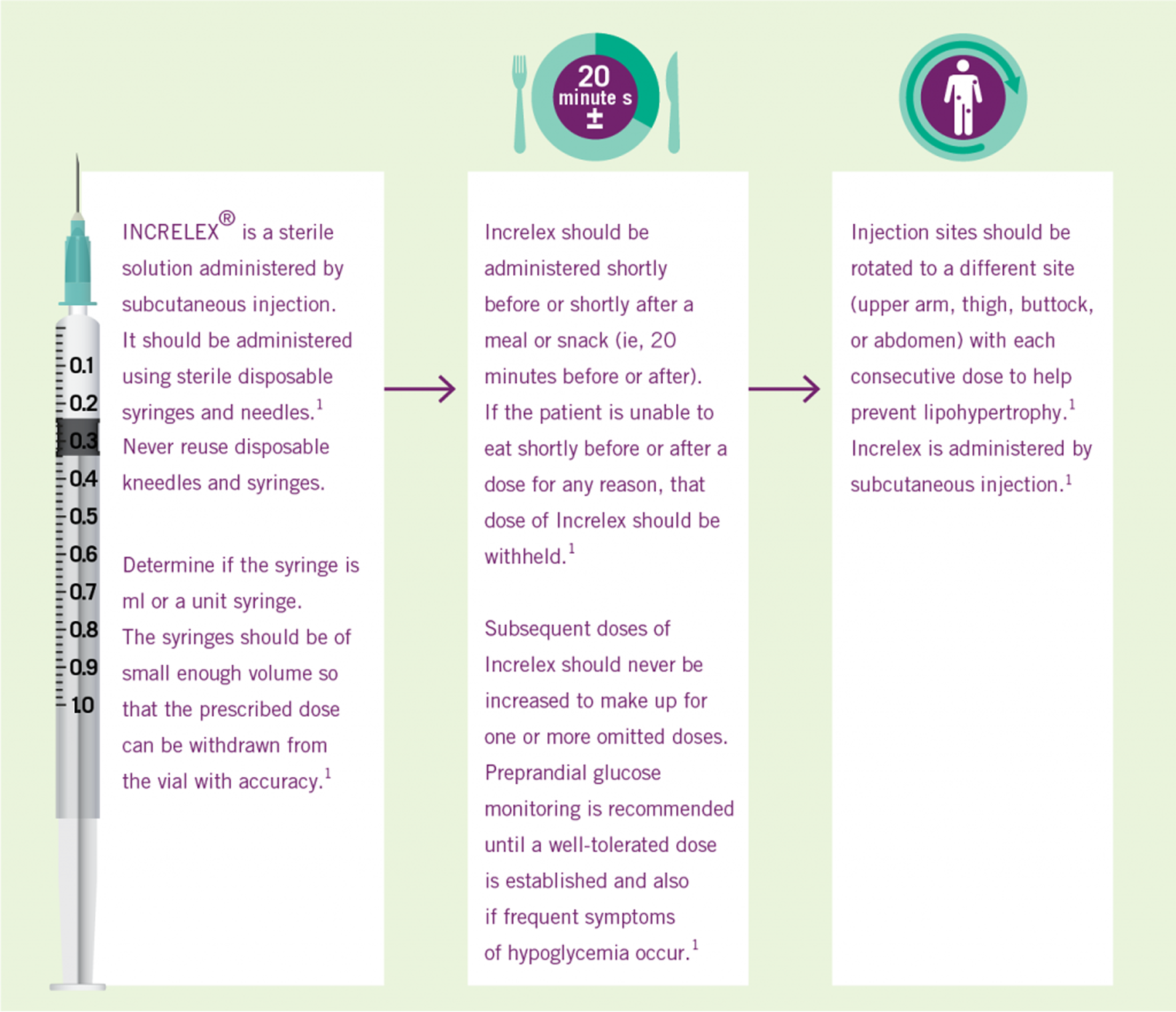
INCRELEX® Administration1
INCRELEX® Supply
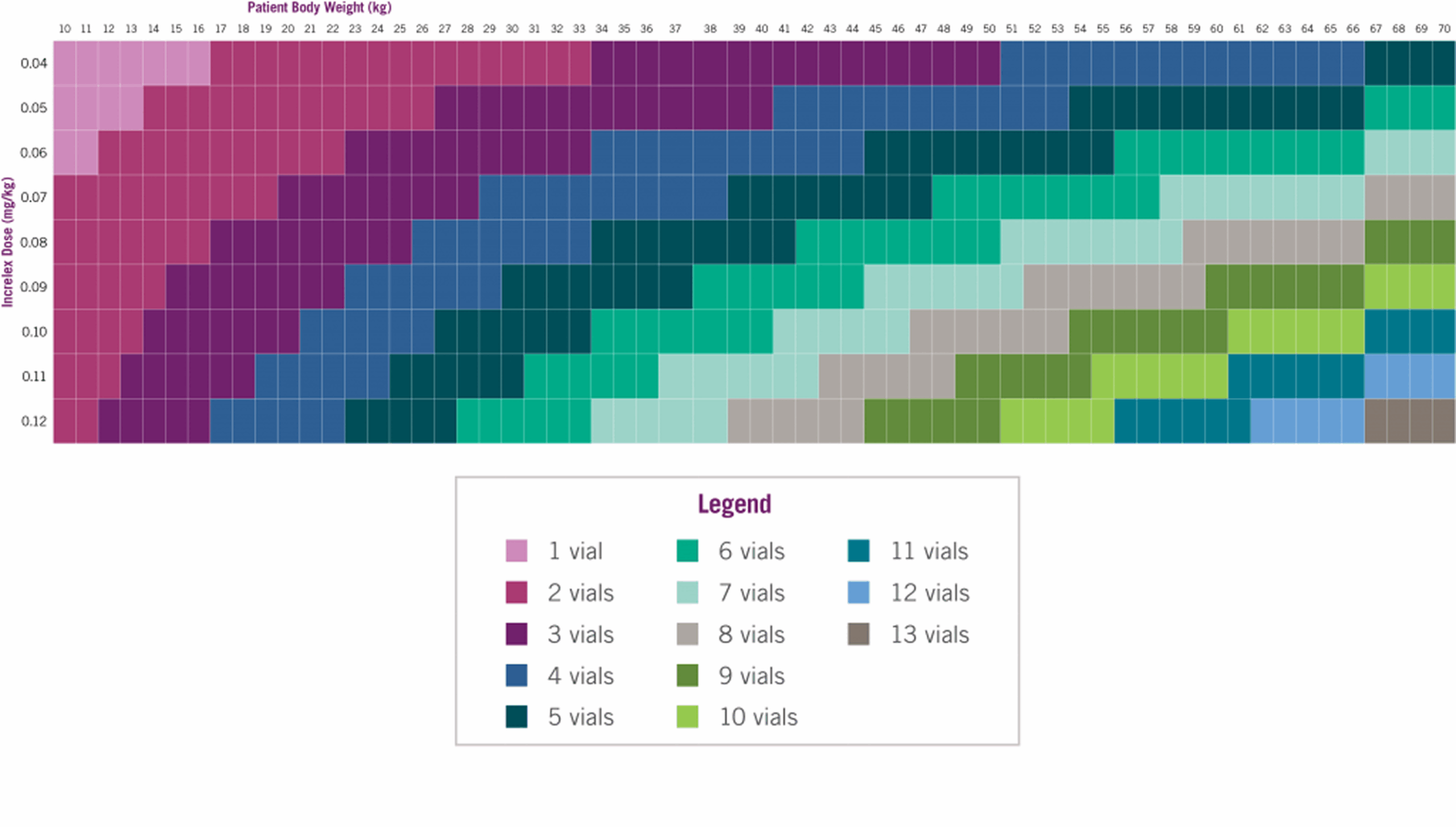
Number of INCRELEX® vials to prescribe for a 30-day supply*4
Indication and important safety information
INCRELEX® (mecasermin) is indicated for the treatment of growth failure in pediatric patients aged 2 years and older with severe primary IGF-1 deficiency* (IGFD), or with hormone (GH) gene deletion who have developed neutralizing antibodies to GH.
Limitations of use: INCRELEX is not a substitute to GH for approved GH indications. INCRELEX is not indicated for use in patients with secondary forms of IGFD, such as GH deficiency, malnutrition, hypothyroidism, or chronic treatment with pharmacologic doses of anti-inflammatory steroids.
*Severe primary IGF-1 deficiency (IGFD) is defined by height standard deviation score ≤ -3.0 and basal IGF-1 standard deviation score ≤ -3.0 and normal or elevated GH.
Important safety information
Contraindications
- Hypersensitivity: to mecasermin (rhIGF-1), any of the inactive ingredients in INCRELEX, or who have experienced a severe hypersensitivity to INCRELEX. Allergic reactions have been reported, including anaphylaxis requiring hospitalization.
- Closed Epiphyses
- Malignant Neoplasia in pediatric patients with malignant neoplasia or a history of malignancy.
Warning and precautions
- Hypoglycemia: INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is omitted. Glucose monitoring and INCRELEX dose titration are recommended until a well-tolerated dose is established and as medically indicated.
- Intracranial Hypertension: Funduscopic examination is recommended at the initiation of and periodically during the course of therapy.
- Lymphoid Tissue Hypertrophy: Patients should have periodic examinations to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients receiving products indicated to treat growth failure and/or short stature, including INCRELEX. Carefully evaluate any pediatric patient with the onset of a limp or hip/knee pain during INCRELEX therapy.
- Progression of Scoliosis: Patients with a history of scoliosis, treated with INCRELEX, should be monitored.
- Malignant Neoplasia: There have been postmarketing reports of malignant neoplasia in pediatric patients who received treatment with INCRELEX. It is unknown whether there is any relationship between Increlex therapy and new occurrences of neoplasia. The tumors were observed more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced serum IGF-1 levels above the normal reference ranges for age and sex. Monitor all patients receiving INCRELEX carefully for development of neoplasms. If malignant neoplasia develops, discontinue INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
Adverse reactions
Common adverse reactions include hypoglycemia, local and systemic hypersensitivity, and tonsillar hypertrophy.
To report a suspected adverse event related to INCRELEX, contact Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
References
- INCRELEX. Package insert. Eton Pharmaceuticals, Inc;2023.
- Cohen J, Blethen S, Kuntze J, et al. Managing the child with severe primary insulin-like growth factor-1 deficiency (IGFD):IGFD diagnosis and management. Drugs R D. 2014;141:25-29.
- Backeljauw PF, Chernausek SD. Treatment of severe IGF-1 deficiency with recombinant human IGF-1 (mecasermin). Curr Med Lit Growth. 2009;23:69-95.
- Data on file. September 2018. Ipsen Biopharmaceuticals, Inc.
Indication and important safety information
INCRELEX® (mecasermin) is indicated for the treatment of growth failure in pediatric patients aged 2 years and older with severe primary IGF-1 deficiency* (IGFD), or with hormone (GH) gene deletion who have developed neutralizing antibodies to GH.
Limitations of use: INCRELEX is not a substitute to GH for approved GH indications. INCRELEX is not indicated for use in patients with secondary forms of IGFD, such as GH deficiency, malnutrition, hypothyroidism, or chronic treatment with pharmacologic doses of anti-inflammatory steroids.
*Severe primary IGF-1 deficiency (IGFD) is defined by height standard deviation score ≤ -3.0 and basal IGF-1 standard deviation score ≤ -3.0 and normal or elevated GH.
Important safety information
Contraindications
- Hypersensitivity: to mecasermin (rhIGF-1), any of the inactive ingredients in INCRELEX, or who have experienced a severe hypersensitivity to INCRELEX. Allergic reactions have been reported, including anaphylaxis requiring hospitalization.
- Closed Epiphyses
- Malignant Neoplasia in pediatric patients with malignant neoplasia or a history of malignancy.
Warning and precautions
- Hypoglycemia: INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is omitted. Glucose monitoring and INCRELEX dose titration are recommended until a well-tolerated dose is established and as medically indicated.
- Intracranial Hypertension: Funduscopic examination is recommended at the initiation of and periodically during the course of therapy.
- Lymphoid Tissue Hypertrophy: Patients should have periodic examinations to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients receiving products indicated to treat growth failure and/or short stature, including INCRELEX. Carefully evaluate any pediatric patient with the onset of a limp or hip/knee pain during INCRELEX therapy.
- Progression of Scoliosis: Patients with a history of scoliosis, treated with INCRELEX, should be monitored.
- Malignant Neoplasia: There have been postmarketing reports of malignant neoplasia in pediatric patients who received treatment with INCRELEX. It is unknown whether there is any relationship between Increlex therapy and new occurrences of neoplasia. The tumors were observed more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced serum IGF-1 levels above the normal reference ranges for age and sex. Monitor all patients receiving INCRELEX carefully for development of neoplasms. If malignant neoplasia develops, discontinue INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
Adverse reactions
Common adverse reactions include hypoglycemia, local and systemic hypersensitivity, and tonsillar hypertrophy.
To report a suspected adverse event related to INCRELEX, contact Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
References
- INCRELEX. Package insert. Eton Pharmaceuticals, Inc;2023.
- Cohen J, Blethen S, Kuntze J, et al. Managing the child with severe primary insulin-like growth factor-1 deficiency (IGFD):IGFD diagnosis and management. Drugs R D. 2014;141:25-29.
- Backeljauw PF, Chernausek SD. Treatment of severe IGF-1 deficiency with recombinant human IGF-1 (mecasermin). Curr Med Lit Growth. 2009;23:69-95.
- Data on file. September 2018. Ipsen Biopharmaceuticals, Inc.
