


Diagnosing SPIGFD
Please see Indication and Important Safety Information below.
On this page

Olive, a former INCRELEX patient, at age 18
Diagnosis of Severe Primary IGF-1 Deficiency
Endocrine Assessment of a Patient With Short Stature
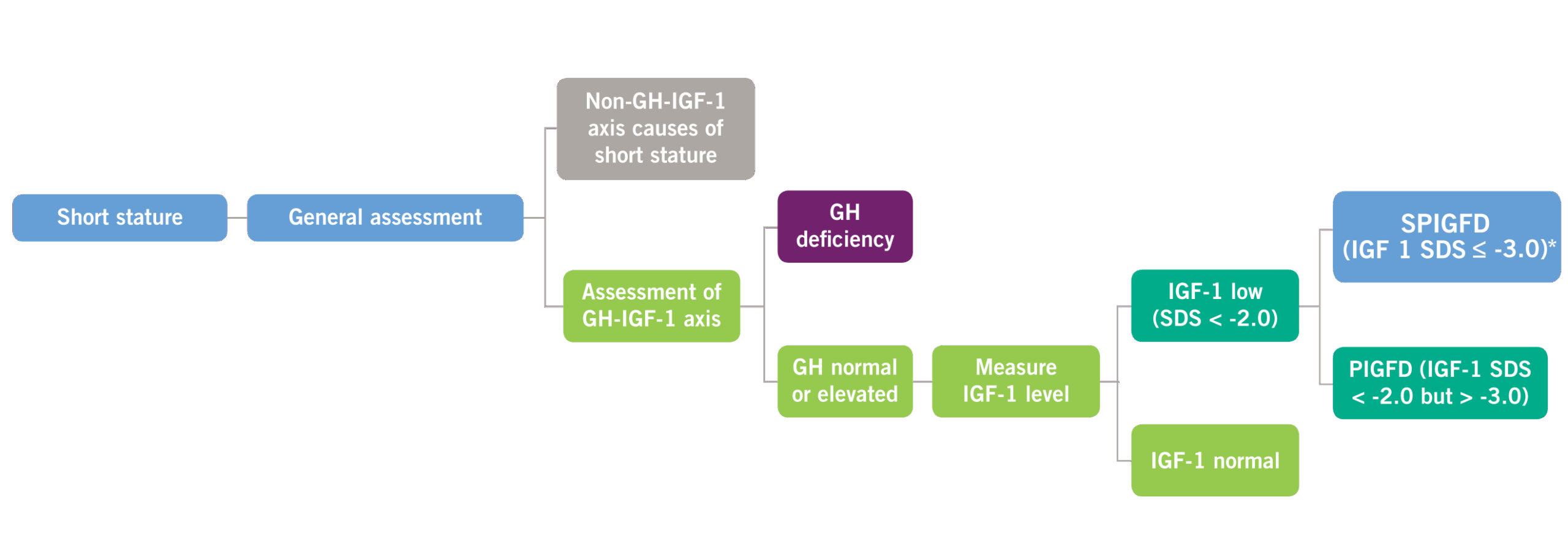
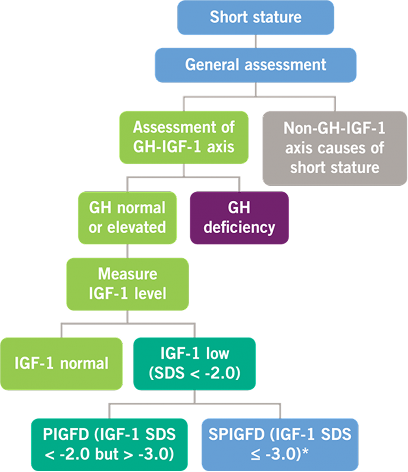
Possible Defects in GH and IGF-1 Levels2,4
GH, growth hormone; IGF-1, insulin-like growth factor-1; PIGFD, primary IGF-1 deficiency; SPIGFD, severe primary IGF-1 deficiency; SDS, standard deviation score. *Defined by a height of SDS ≤ -3.0, basal IGF-1 SDS ≤ -3.0, and normal or elevated GH.
Clinical Presentations of Primary IGFD
- Hypoglycemia
- Obesity (with abdominal adiposity)
- Undeveloped muscles
- Midfacial hypoplasia
- High-pitched voice
- Osteoporosis
- Small genitalia since birth
- Delayed puberty
Criteria for a Diagnosis of SPIGFD
The diagnosis of SPIGFD is made when a patient meets the following criteria:
- Height standard deviation score (SDS) ≤ -3.0
- IGF-1 concentration SDS ≤ -3.0
- GH is normal or elevated
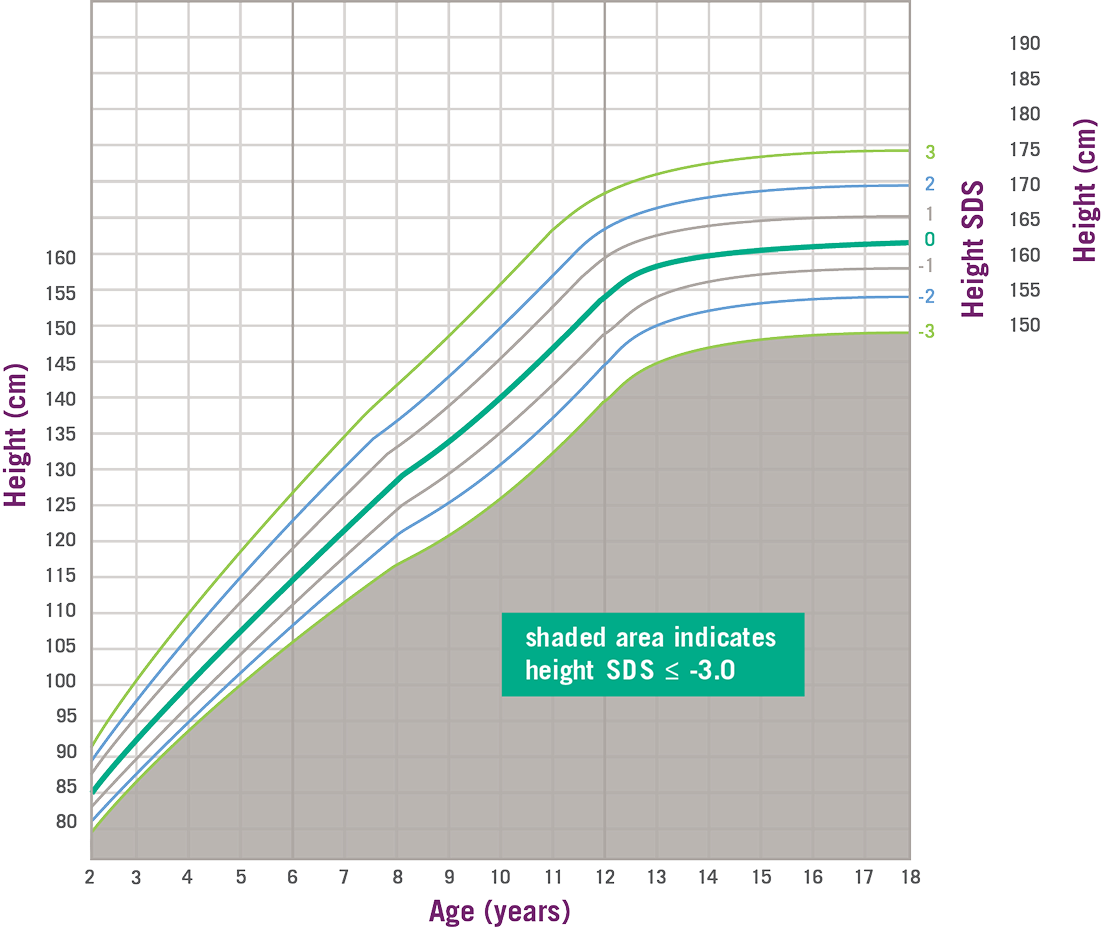
SPIGFD Patients Have Height SDS of ≤ -3.0*2
The Importance of a Proper Diagnosis
Assessment of the GH–IGF-1 Axis
- GH provocation (stimulation) test
- Circulation levels of GH binding protein (GHBP)
- IGF-1 generation test
- Genetic analysis of the IGF-1/GH axis
Monitoring Your Patients During the Course of Treatment
In children previously diagnosed with a growth disorder and treated with GH, treatment response may vary by diagnosis, as well as by age and gender. If a child’s growth velocity is tracking below the average on an appropriate growth chart, this may be an indication that additional evaluation or re-evaluation is necessary. This evaluation may include an assessment of compliance, and whether other diagnoses, including SPIGFD, should be considered.
Indication and important safety information
INCRELEX® (mecasermin) is indicated for the treatment of growth failure in pediatric patients aged 2 years and older with severe primary IGF-1 deficiency* (IGFD), or with hormone (GH) gene deletion who have developed neutralizing antibodies to GH.
Limitations of use: INCRELEX is not a substitute to GH for approved GH indications. INCRELEX is not indicated for use in patients with secondary forms of IGFD, such as GH deficiency, malnutrition, hypothyroidism, or chronic treatment with pharmacologic doses of anti-inflammatory steroids.
*Severe primary IGF-1 deficiency (IGFD) is defined by height standard deviation score ≤ -3.0 and basal IGF-1 standard deviation score ≤ -3.0 and normal or elevated GH.
Important safety information
Contraindications
- Hypersensitivity: to mecasermin (rhIGF-1), any of the inactive ingredients in INCRELEX, or who have experienced a severe hypersensitivity to INCRELEX. Allergic reactions have been reported, including anaphylaxis requiring hospitalization.
- Closed Epiphyses
- Malignant Neoplasia in pediatric patients with malignant neoplasia or a history of malignancy.
Warning and precautions
- Hypoglycemia: INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is omitted. Glucose monitoring and INCRELEX dose titration are recommended until a well-tolerated dose is established and as medically indicated.
- Intracranial Hypertension: Funduscopic examination is recommended at the initiation of and periodically during the course of therapy.
- Lymphoid Tissue Hypertrophy: Patients should have periodic examinations to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients receiving products indicated to treat growth failure and/or short stature, including INCRELEX. Carefully evaluate any pediatric patient with the onset of a limp or hip/knee pain during INCRELEX therapy.
- Progression of Scoliosis: Patients with a history of scoliosis, treated with INCRELEX, should be monitored.
- Malignant Neoplasia: There have been postmarketing reports of malignant neoplasia in pediatric patients who received treatment with INCRELEX. It is unknown whether there is any relationship between Increlex therapy and new occurrences of neoplasia. The tumors were observed more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced serum IGF-1 levels above the normal reference ranges for age and sex. Monitor all patients receiving INCRELEX carefully for development of neoplasms. If malignant neoplasia develops, discontinue INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
Adverse reactions
Common adverse reactions include hypoglycemia, local and systemic hypersensitivity, and tonsillar hypertrophy.
To report a suspected adverse event related to INCRELEX, contact Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
References
- Cohen J, Blethen S, Kuntze J, et al. Managing the child with severe primary insulin-like growth factor-1 deficiency (IGFD): IGFD diagnosis and management. Drugs R D. 2014;141:25-29.
- INCRELEX. Package insert. Eton Pharmaceuticals, Inc;2023.
- Cohen P, Rogol AD, Deal CL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;9311:4210-4217.
- Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone IGF-I axis defects causing short stature: diagnostic and therapeutic challenges. Clin Endocrinol (Oxf). 2010;726:721-728.
- Fintini D, Brufani C, Cappa M. Profile of mecasermin for the long-term treatment of growth failure in children and adolescents with severe primary IGF-1 deficiency. Ther Clin Risk Manag. 2009;53:553-559.
- Wit JM, Kiess W, Mullis P. Genetic evaluation of short stature. Best Pract Res Clin Endocrinol Metab. 2011;251:1-17.
- Grimberg A, DiVall S, Polychronakos C, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86:361-397.
Indication and important safety information
INCRELEX® (mecasermin) is indicated for the treatment of growth failure in pediatric patients aged 2 years and older with severe primary IGF-1 deficiency* (IGFD), or with hormone (GH) gene deletion who have developed neutralizing antibodies to GH.
Limitations of use: INCRELEX is not a substitute to GH for approved GH indications. INCRELEX is not indicated for use in patients with secondary forms of IGFD, such as GH deficiency, malnutrition, hypothyroidism, or chronic treatment with pharmacologic doses of anti-inflammatory steroids.
*Severe primary IGF-1 deficiency (IGFD) is defined by height standard deviation score ≤ -3.0 and basal IGF-1 standard deviation score ≤ -3.0 and normal or elevated GH.
Important safety information
Contraindications
- Hypersensitivity: to mecasermin (rhIGF-1), any of the inactive ingredients in INCRELEX, or who have experienced a severe hypersensitivity to INCRELEX. Allergic reactions have been reported, including anaphylaxis requiring hospitalization.
- Closed Epiphyses
- Malignant Neoplasia in pediatric patients with malignant neoplasia or a history of malignancy.
Warning and precautions
- Hypoglycemia: INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is omitted. Glucose monitoring and INCRELEX dose titration are recommended until a well-tolerated dose is established and as medically indicated.
- Intracranial Hypertension: Funduscopic examination is recommended at the initiation of and periodically during the course of therapy.
- Lymphoid Tissue Hypertrophy: Patients should have periodic examinations to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis may lead to osteonecrosis. Cases of slipped capital femoral epiphysis with or without osteonecrosis have been reported in pediatric patients receiving products indicated to treat growth failure and/or short stature, including INCRELEX. Carefully evaluate any pediatric patient with the onset of a limp or hip/knee pain during INCRELEX therapy.
- Progression of Scoliosis: Patients with a history of scoliosis, treated with INCRELEX, should be monitored.
- Malignant Neoplasia: There have been postmarketing reports of malignant neoplasia in pediatric patients who received treatment with INCRELEX. It is unknown whether there is any relationship between Increlex therapy and new occurrences of neoplasia. The tumors were observed more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced serum IGF-1 levels above the normal reference ranges for age and sex. Monitor all patients receiving INCRELEX carefully for development of neoplasms. If malignant neoplasia develops, discontinue INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
Adverse reactions
Common adverse reactions include hypoglycemia, local and systemic hypersensitivity, and tonsillar hypertrophy.
To report a suspected adverse event related to INCRELEX, contact Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
References
- Cohen J, Blethen S, Kuntze J, et al. Managing the child with severe primary insulin-like growth factor-1 deficiency (IGFD): IGFD diagnosis and management. Drugs R D. 2014;141:25-29.
- INCRELEX. Package insert. Eton Pharmaceuticals, Inc;2023.
- Cohen P, Rogol AD, Deal CL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;9311:4210-4217.
- Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone IGF-I axis defects causing short stature: diagnostic and therapeutic challenges. Clin Endocrinol (Oxf). 2010;726:721-728.
- Fintini D, Brufani C, Cappa M. Profile of mecasermin for the long-term treatment of growth failure in children and adolescents with severe primary IGF-1 deficiency. Ther Clin Risk Manag. 2009;53:553-559.
- Wit JM, Kiess W, Mullis P. Genetic evaluation of short stature. Best Pract Res Clin Endocrinol Metab. 2011;251:1-17.
- Grimberg A, DiVall S, Polychronakos C, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86:361-397.
